TheSamba.com :: Eurovan - View topic - Clockspring Questions - ASR ABS BRAKE (flashing) + 3 loud beeps

Volkswagen Golf GTI Mk IV Headlight and Dimmer Switch Replacement (1999-2005) - Pelican Parts DIY Maintenance Article

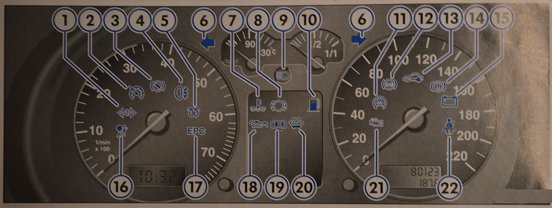
Dashboard Instrument Blue Needle White Background Scale Paper LED Light Retrofitting For VW Bora MK4 Golf 4 R32 Meter 300 - AliExpress

Amazon.com: ZIYOLIGHT PL9140A - LED Interior Light Kit Package Replacement for Volkswagen Golf GTI R32 MK4 1997-2004, Xenon White Dome Light Bulbs Upgrade Error Free (MK4 1997-2004 (11 Bulbs)) : Automotive