adidas Tiro Pro - Guantes de portero unisex para adultos, negro/blanco/ hierro metálico, 7 : Amazon.es: Deportes y aire libre

Amazon.com: adidas X Pro guante unisex para adultos, blanco/hierro metálico/negro/rojo solar, 8 : Todo lo demás

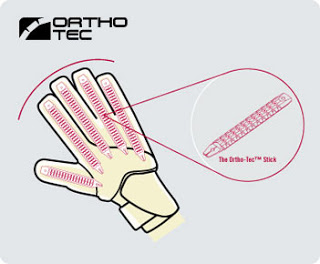
Guantes de portero de fútbol profesionales, guantes de látex con agarre fuerte para niños y adultos, 1 unidad - AliExpress