
Compre 2019 Los Nuevos Sombreros Para Hombres Y Mujeres De Alta Calidad Con Tapa De Bola Ligera Transpirable, Tapa De Piel Con Polvo Y Bolsa A 32,19 € Del Wsj588 | Es.Dhgate.Com
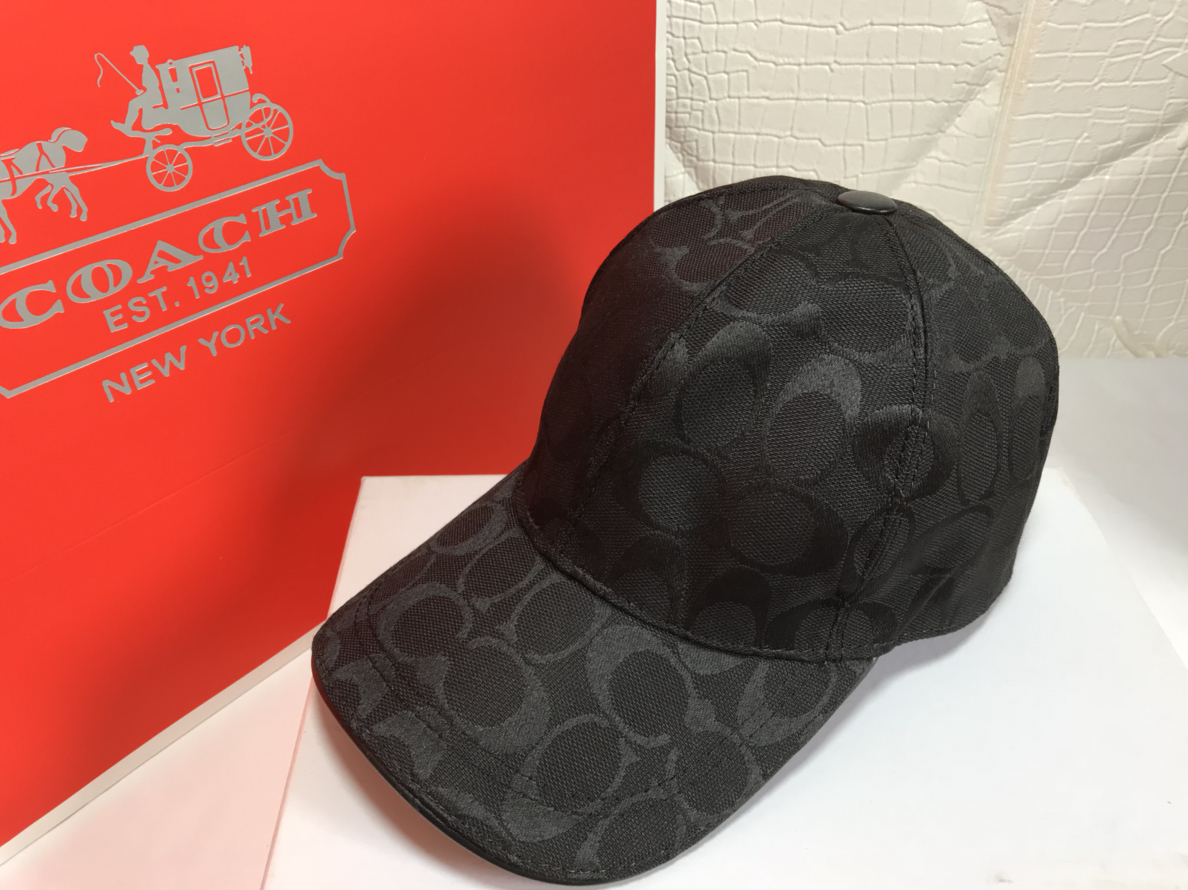
Compra 2019 Los Nuevos Sombreros Para Hombres Y Mujeres De Alta Calidad Con Tapa De Bola Ligera Transpirable Tapa De Cuero Con Clavo Con Bolsa De Polvo Barato | Entrega Rápida Y




















