
RABALUX 2186 | Azra Rabalux mennyezeti lámpa impulzus kapcsoló szabályozható fényerő 1x LED 3200lm 4000K króm, fehér | Mennyezeti lámpák - Lámpa, csillár, világítástechnika - Fénypost - Lámpa rendelés
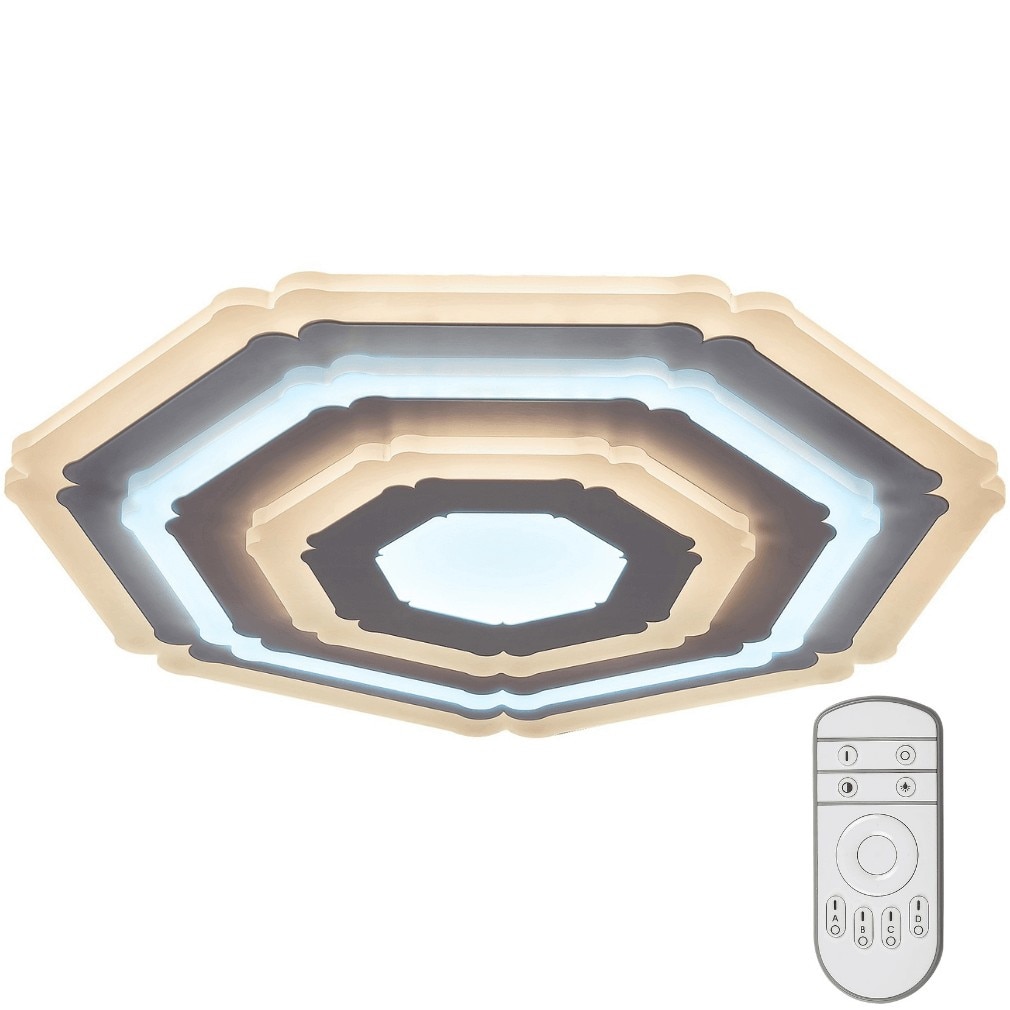
Rabalux Taneli integrált Led mennyezeti lámpa, távirányítóval, 41W, 3046 lm, állítható fényhőmérséklet, szabályozható, IP20, 500 mm, fehér - eMAG.hu

RÁBALUX 2764 OSCAR LED Mennyezeti lámpa 18W, 1350LM, szögletes fa - ár, vásárlás, rendelés, vélemények

Rabalux 1510 - LED Szabályozható mennyezeti lámpa távirányítóval LEONIE RGB LED/32W/230V | lampak.hu

Vásárlás: Rábalux Beltéri led mennyezeti lámpa 40w 3200lm 3000-6500k fehér willie (2105) Fali- és mennyezeti lámpa, csillár árak összehasonlítása, Beltéri led mennyezeti lámpa 40 w 3200 lm 3000 6500 k fehér willie 2105 boltok


















